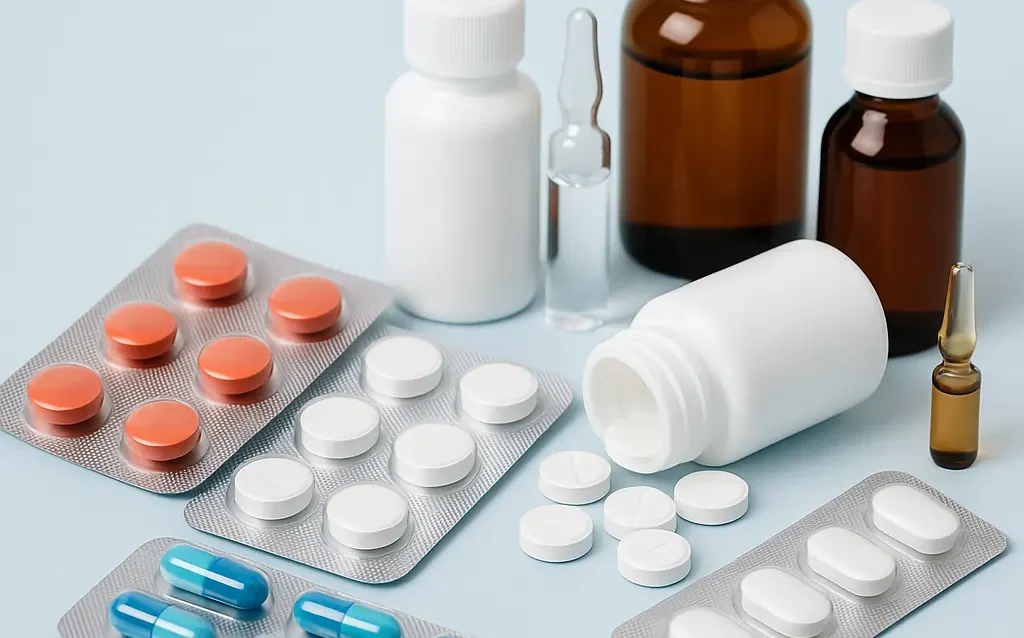
Every medicine that reaches patients comes in a ready-to-use form—called a Finished Dosage Formulation (FDF). These include tablets, capsules, injections, syrups, and creams. At Ramoliya Pharmaceuticals, we manufacture and export a wide portfolio of Finished Dosage Formulations, ensuring global compliance, affordability,…
API GMP Checklist: CoA, MSDS, Stability & Supplier Audits
When sourcing Active Pharmaceutical Ingredients (APIs), it’s not just about cost or availability—quality and compliance are critical. A single weak link in the supply chain can compromise patient safety and regulatory approval. At Ramoliya Pharmaceuticals, we follow a strict API GMP…
USP vs EP vs IP vs BP: Pharmacopoeia Differences Explained
Introduction Pharmaceutical quality standards are defined by pharmacopoeias—official reference books that describe how medicines and ingredients should be tested, measured, and standardized. At Ramoliya Pharmaceuticals, we work with global partners and ensure our 250+ APIs and Finished Formulations comply with leading…
What Are Active Pharmaceutical Ingredients (APIs)? A Complete Guide
Introduction In the world of medicine, every tablet, capsule, or injection starts with one crucial component: the Active Pharmaceutical Ingredient (API). APIs are the biologically active parts of medicines that produce the intended therapeutic effect. At Ramoliya Pharmaceuticals, we specialize in…